HLTH Europe: DTx Regulation, Generative AI and Women's Health
HLTH is a popular digital health event in the US market, attracting 12,000 attendees in 2023. In 2024, it made its European debut, drawing 400 speakers and 3,500 participants. What resonated?
This is a newsletter of Faces of Digital Health - podcast that explores the diversity of healthcare systems and healthcare innovation worldwide. Interviews with policymakers, entrepreneurs, and clinicians provide the listeners with insights into market specifics, go-to-market strategies, barriers to success, characteristics of different healthcare systems, challenges of healthcare systems, and access to healthcare. Find out more on the website, tune in on Spotify or iTunes.
It’s almost impossible to summarize a conference that had more than 6 stages (Main, Sound, Sight, Health, Touch, Aroma, Forum, Startup …), coupled with tons of parallel events and partner programming. You can access recordings of all sessions on the HLTH Community platform.
The topics below are only a fraction of the content presented at HLTH Europe.
Art Gallery at HLTH Europe showcased art installations from three patient groups from across Europe, alongside digital submissions from clinicians, patients and people working in health.
Europe: Diverse and Fragmented
When examining the European healthcare market from a business perspective, one finds few positive connotations: the market is fragmented. Every country is different, each insisting on its own specific regulations. Do you speak English? Great, but in Germany, you’ll need a local German-speaking team member. In France, ensure you have a French-speaking team. In Switzerland, be prepared to offer your solution in four languages. On the positive side, healthcare is relatively accessible. However, even this is under increasing sustainability pressure – in the NHS, 8 million people are currently (data from April 2024) waiting for hospital treatment.
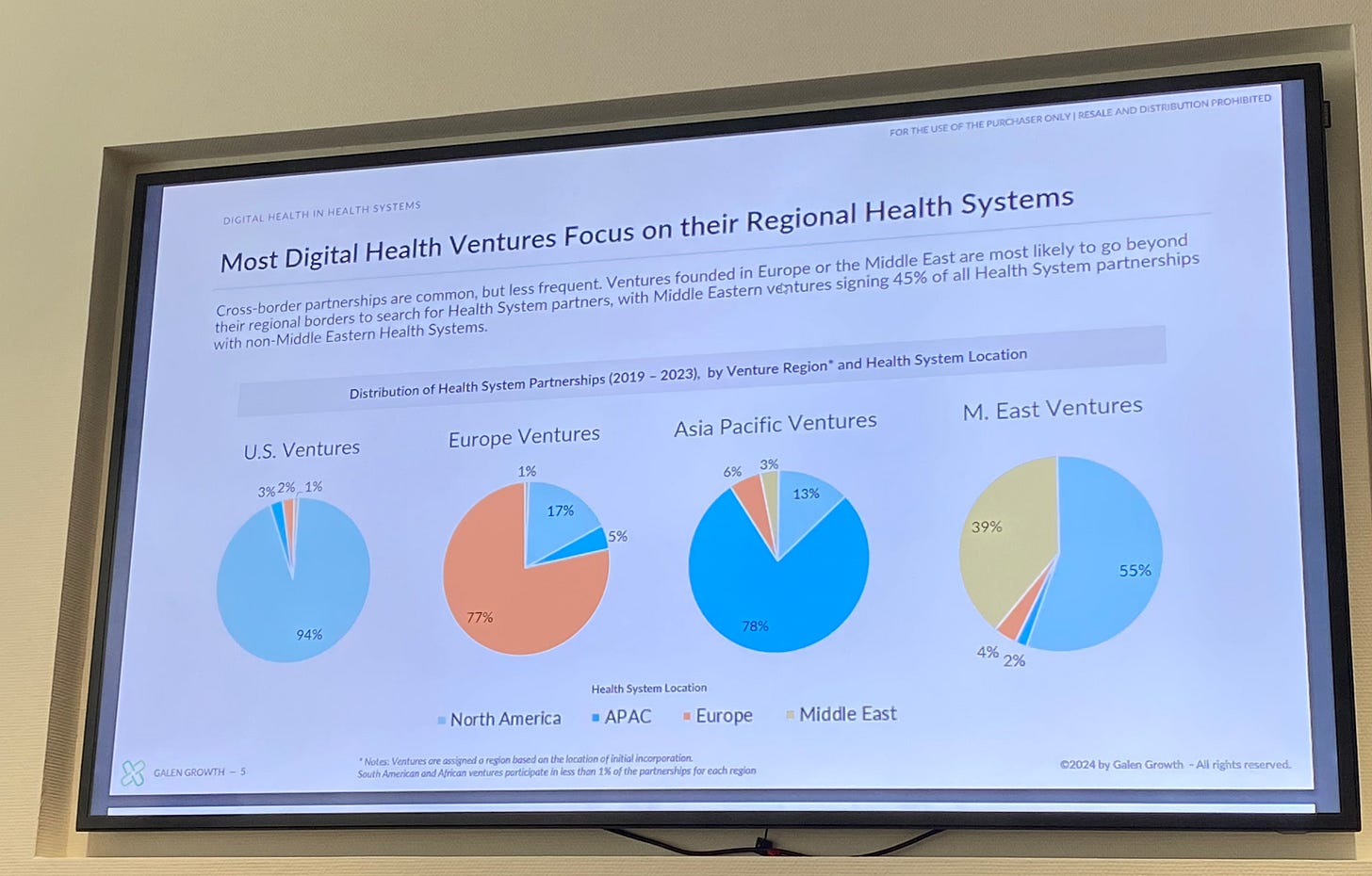
Despite these challenges, the European values of solidarity and healthcare accessibility remain strong. Governments are investing in digitalization as a means to improve efficiency and sustainability, and startup founders still aspire to heal the market with their innovative ideas. Most companies in Europe still focus on the European market, with 17% aiming at the U.S., according to Sara Schmachtenberg, Digital Health Venture Expert and Head of Data and Analytics for Europe & USA at Galen Growth.
DiGA, PECAN, here we come!
At HLTH Europe, a hot topic was the reimbursement and regulation of digital therapeutics. How does one become a DiGA app in Germany? If you successfully undergo the approval process with the regulator BfArM, your solution needs to be reimbursed by insurance companies. As of June 2024, there are just over 50 apps in the national repository, a possibility available since 2019.
In 2023, France unveiled their PECAN program, a new reimbursement pathway for digital therapeutics and remote monitoring solutions. By June 2024, one solution for telemonitoring was approved. Reimbursement rates for DTx were defined in April this year and will range between 435 EUR and 780 EUR in the first year after approval and will be reassessed later.
6-7 solutions are expected to be approved by the end of 2024 through PECAN, said Stephane Tholander, Co-founder of AppTherra - the digital therapeutics distribution platform.
Getting through the approval process is loooong. In Germany, it never takes less than a year and a half, said Dorothée Marie-Louise Doepfer, Deputy Head of Digital Health Accelerator at the Berlin Institute of Health at Charité, who chaired a session on the German market.
If certification seems demanding, getting through the approval process is actually the easy part of a DTx company’s journey. People in the digital health space may know what digital therapeutics are, but most clinicians don’t. Getting your distribution channels right and educating doctors about the clinical validity and usability of your solution is the real Herculean task. Oh… And then the doctors also need to educate patients about the proper use of these new treatments. If implementation is 60% of success in digital transformation, the same is probably true for patient education and the success of digital therapeutics.
EU AI Act, EHDS and credibility scores for generative AI
Most of the discussions around AI stayed high-level and focused on the potentials of technology. Hype around generative AI has settled down in 2024, with the ongoing race, especially in the US market, around which will be the number one clinical note taking and summarization solution provider.
According to Galen Growth, by the end of 2023, 2% of all digital health ventures were using generative AI. AI more broadly is a different case with over 50% were using some form of AI.
In a short discussion (which will be published as a podcast in July), John Halamka, President of Mayo Clinic Platform shared his view on the European regulation of AI and data, the latest on generative AI testing and cross-border collaboration for data exchange.
In short: GDPR is not a restriction on the secondary use of data, it’s a guideline on “the how”, he says.
In the field of generative AI regulation, companies are beginning to develop credibility and quality measures for generative AI. Since the quality of AI-generated answers can fluctuate, assigning a numeric credibility score will help determine when an answer is reliable or if further prompting is necessary.
Generative AI will always produce some inaccurate and subpar responses, posing varying levels of risk depending on the use case. Hence healthcare requires consensus on what this technology should or shouldn’t be used for, says Halamka. For instance, using AI to summarize historical records is low-risk, while allowing it to decide on treatment plans is high-risk and potentially harmful. Therefore, prioritizing low-risk, high-yield administrative use cases is recommended.
He sees the Netherlands, Sweden and Denmark as the European frontrunners in their willingness to take risks and implement AI.
Girls, Ladies, we need to invest
One thing that got noticed by many at the conference, was a dedicated women’s health hub, standing at the Heart stage in the middle of the show floor.
Women’s health was front and center, emerging as a key topic set to shape digital health in 2024. This focus is unsurprising given that 80% of patients with autoimmune diseases and 66% of Alzheimer’s patients are women. Women also experience a higher incidence of migraines, and cardiovascular diseases present differently in women than in men. Many of these statistics still lack clear explanations.
“Women’s health is not a niche. 55% of the population is not a niche. So don’t invest in women’s health because it’s the right thing to do, but because it’s the smart thing to do,” said Priya Agrawal, VP of Health Equity & Partnerships at MSD, during a panel on women’s health, highlighting the significant business potential in this field.
The discussions and booths covered a wide range of topics, including methods for gathering DNA after a sexual assault (women have a 72-hour window to collect DNA from the perpetrator, which can be crucial evidence in court), sanitary tools for use after intercourse, wearable devices with sensors to monitor fetal health during pregnancy, wellness at-home blood test kits, and more.
“We put the patient at the center”
…says almost every company targeting patients... Yet, they often forget to include patients in the discovery process and product design. By only involving patients after the final product is ready, companies shouldn't be surprised if patients find their solutions useless.
This is what Jen Horonjeff, PhD, Founder and CEO of Savvy Cooperative, and Ronnie Sharpe, co-founder and COO at Savvy Cooperative, say one shouldn’t forget about patients and product development:
Patient-Centered Outcomes: Involve patients from the start in drug development to determine which outcomes and endpoints matter most to them, and iterating on their experiences throughout the trial and commercialization phases.
Feedback and Co-Design: Incorporate patient feedback in the development of digital health tools, avoiding the assumption that sponsors or doctors know best, and ensuring tools are co-designed with patients to meet their needs effectively.
Integration of Tools: Ensure that digital tools integrate seamlessly with each other and other devices patients use, to avoid adding frustration and burden from managing multiple, disconnected tools.
Access to Data: Provide patients with access to their data, as many track their health using various devices, and addressing concerns about data sharing to empower patients in managing their health.
Iterative Development: Utilize usage data to inform the iterative development of digital tools, engaging with users to refine and improve tools rather than abandoning them if initial usage is low, similar to product development in other sectors.
If you’re a podcast fan, tune in to the HLTH Europe recap episode with some of the speakers mentioned above and others:
John Halamka, President of Mayo Clinic, about AI regulation and future,
Sara Schmachtenberg, Digital Health Venture Expert / Head of Data and Analytics, Europe & USA at Galen Growth, about who ventures partner with,
Simon Phillip Rost, the Chief Marketing Officer at GE Healthcare, about patient engagement,
Jen Horonjeff, PhD, Founder and CEO of Savvy Cooperative, and Ronnie Sharpe, co-founder and COO at Savvy Cooperative, about including patients in product development,
Christophe Jauquet, Author & Professional Keynote Speaker on how business & technology shape a healthier, happier, more sustainable future, about values to consider when designing for digital health,
Dorothée Marie-Louise Doepfer, Deputy Head of Digital Labs / Program Management Digital Health Accelerator & Community Building at Berlin Institute of Health at Charité, about women’s health and entering the German market.
This is it for this edition. Feedback is always welcome, feel free to share your thoughts in a comment or email.
Enjoy the summer!
Before you leave…
🎥 🎞️ [VIDEO] 🎥 🎞️
Head to Youtube to hear the following discussions:
➡ John Halamka on AI in healthcare, regulation and country-specific differences.
➡ Sara Schmachtenberg, Galen Growth | Insights You Can Trust about who digital health companies partner with, and which markets different continents focus on.
➡ Zayna Khayat, Ph.D. and Lucien Engelen about their observation of carification of retail, phlebotomy automation, hope and upstream health.
➡ Andrew Lacy, CEO of Prenuvo, about influencer marketing, whole-body MRI scans and prevention.
➡ Peter Birch, Host of Talking HealthTech and Dr. James Somauroo, Host of The Healthtech Podcast about LGBTQ healthcare, women's health, communities and social media and healthcare.